Vital Transformation is a small, unique consultancy focused on addressing the challenges of today’s modern healthcare system. We partner with your organisation to help you find answers to hard to solve problems.
Contact us to learn how Vital Transformation can help your organisation.
LATEST COMMUNICATIONS

Our Communication Services
John LaMattina was President of Pfizer Global Research and Development and ran an international team of over 13,000 scientists and professionals. He has authored several books, including the highly acclaimed Pharma and Profits – Balancing Innovation, Medicine, and Drug Prices. He is also a senior partner at PureTech Health and a contributor to Forbes.
Last year, when John was a guest on the Vital Health Podcast, the potential impacts of the Inflation Reduction Act (IRA) were still theoretical. But one year on, the Centers for Medicare and Medicaid Services (CMS) released their prices for drug negotiations, and the impacts of the IRA have now become real. John discusses how the IRA will impact the development of critically needed new therapies and provides his insights into the Biden Administration’s recent threats to use march-in rights to confiscate intellectual property as a way to control the price of drugs.
Find all our communication projects on Better Science, Better Health
MORE RESEARCH
The Impact of The House Proposed IRA Expansion on the US Biopharma Ecosystem
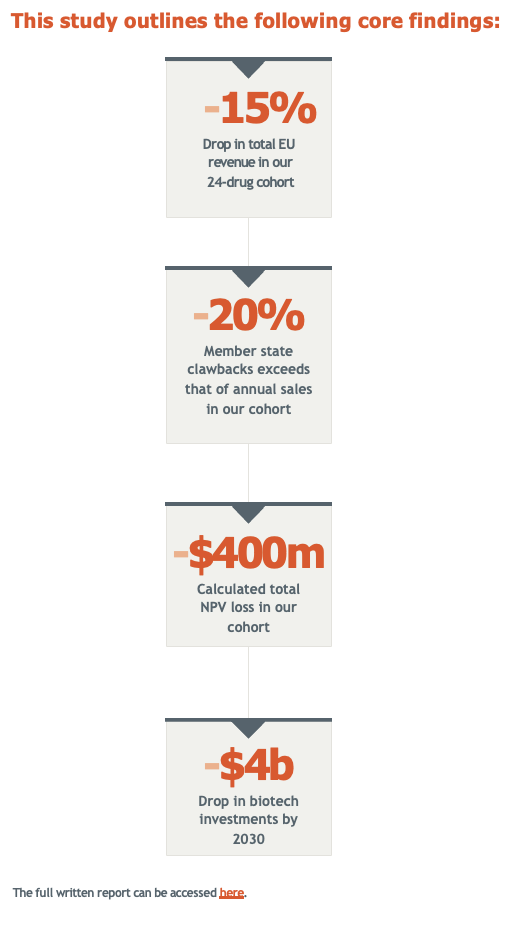
Vital Transformation (VT) modeled the impacts of the drug pricing provisions of the Inflation Reduction Act, with the expansions proposed by Representative Frank Pallone, Jr. (NJ-06) called the, “H.R. 4895: Lowering Drug Costs for American Families Act”, which would impose government price setting for up to 50 selected Medicare Drugs starting in 2029 and expand those negotiated prices to the commercial market.
We modeled the impacts on industry revenues, future R&D investments for the Medicare aged population, and lost innovation including industry jobs.
We estimate a loss of 136,000 – 216,000 direct biopharmaceutical industry jobs and 678,000 – 1,076,000 indirect jobs across the U.S. economy if H.R. 4895 were to be implemented.
We estimate that the expanded government price setting could result in roughly 134 fewer FDA approvals of new medicines treating primarily the Medicare aged population over a ten-year period:
- Impacts will be felt most heavily in many areas of unmet need, including in rare disease, oncology, neurology, and infectious disease targeting those over 65 years of age.
- H.R. 4895 impacts the entire commercial market at the point of negotiated prices entering Medicare.
- The most significant ecosystem impacts would be concentrated primarily in CA and MA.
- Had the drug pricing provisions of the House Bill H.R. 4895 been in place prior to the development of today’s top-selling medicines, we estimate that 76 of the 198 therapies we identified as selected for Medicare price setting would likely have not been developed.
March-in rights under the Bayh-Dole Act & NIH contributions to pharmaceutical patents
Industry is the dominant source of innovation for novel FDA approved medicines; industry funding for novel patented therapies approved by the FDA from 2011 – 2020 was $44.3 billion, compared to the $276 million the US government contributed to patented drugs that are subject to Bayh Dole. Relevant to this, the previously published report by VT found that over the 20 years leading up to FDA approval of 18 innovative new therapies, the funding contribution from industry to the development of these therapies was 66 times higher than the public funding from all linked NIH grants.
Research and development investments are a significant risk to the pharmaceutical industry and their investors as not all assets result in a marketed product. The high rate of failure in drug development underscores the challenges and risks associated with bringing new treatments to patients.
92% of the therapies in our cohort have no mechanism of action or composition of matter patents with a government interest statement or federally funded co-development program in connection to them.
99% of the therapies in our cohort cannot be marched-in upon, as the key patents studied do not cover the entire asset’s intellectual property. There are only 5 out of 361 pharmaceutical products in which all available MoA and CoM patents include a government interest statement and could be subject to march-in rights.
Enacting march-in rights to control prices will create uncertainty and increase risk for inventors based upon previous experiences with fair pricing requirements for NIH CRADAs:
- Industry, venture capital will avoid investing in commercializing academic inventions and partnerships with the NIH will plumet
- US biopharma productivity will drop significantly, providing an opportunity for competing foreign biopharma markets to expand, with a high potential for industry to relocate their enterprises
IRA – Saving Money, or Playing Politics?
IRA’s drug negotiation choices seem to be much more about the 2024 election than Medicare cost savings in 2026.
By Duane Schulthess, John Lamattina, and Harry P. Bowen
September 13th, 2023
On Tuesday August 29th, the Biden Administration and the Centers for Medicare & Medicaid Services (CMS) announced the first 10 drugs to be selected as part of their signature legislation, the Inflation Reduction Act (IRA).
The linchpin of the IRA has been to lower total Medicare costs. The text of the IRA states clearly that the drugs to be chosen for negotiation are those, “… drugs with the highest total expenditures being ranked the highest.” This language is completely unambiguous; drugs with the greatest total expenditures for taxpayers are the ones to be negotiated.
The IRA also includes provisions that drugs soon to be subject to generic or biosimilar competition are excluded from negotiation. This is logical as once a drug loses its patent exclusivity its price falls rapidly.
Our firm, Vital Transformation (VT), has been at the center of the IRA debate for well over a year. Given the IRA’s clear objective to contain taxpayer costs, we conducted economic modeling which simulated the impact of 10 years of IRA negotiations on Medicare spending and biopharmaceutical company revenues.
VITAL HEALTH PODCASTS
Our podcast series is also available via your favourite channels!
 |
 |
|---|---|
 |
 |
NEWSLETTER
Register now to receive all the latest updates from Vital Transformation including our research, podcasts and more…
VT on X
BioCentury’s @steveusdin1 profiles our latest research, finding that the IRA selection list was designed to minimize cancer drugs, and to maximize the number of Medicare beneficiaries before the 2024 elections. https://t.co/EFLDtiejyW @John_LaMattina @duaneschulthess
— VitalTransformation (@VitalTransform) September 14, 2023
Our clients include many of the world’s leading health care organisations.