Vital Transformation is a small, unique consultancy focused on addressing the challenges of today’s modern healthcare system. We partner with your organisation to help you find answers to hard to solve problems.
Contact us to learn how Vital Transformation can help your organisation.
LATEST RESEARCH
LATEST COMMUNICATIONS

Our Communication Services
In this episode of the Vital Health Podcast, host Duane Schulthess speaks with Tim Scott, President & CEO of Biocom California, a biopharma executive with more than two decades of experience, including spinning out companies from UC San Diego and leading firms acquired by BioMarin and Novartis, to discuss how California’s life sciences ecosystem became a global innovation engine, why the state’s research and venture networks matter, and how policy headwinds such as the Inflation Reduction Act (IRA) and Most Favored Nation (MFN) reference pricing reshape investment, rare disease development, and competition with Europe and China.
Find all our communication projects on Better Science, Better Health
MORE RESEARCH
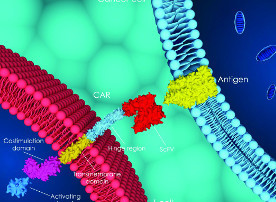
Barriers to CAR T use in the spotlight at first European meeting
Publish date: February 28, 2019 By Sharon Worcester The high cost of chimeric antigen receptor (CAR) T-cell therapy largely limits its use to the sickest patients and prohibits experimentation in “less-diseased” populations, outcomes data suggest.
Don’t Believe the Hype – International Reference Pricing Will Cost Far More than 1% of R&D Budgets
By Duane Schulthess - 26 December 2018
It’s odd that a city like Brussels managed to carve itself out a niche as one of the major political centres of the world. The medieval site of a notoriously typhus infested bog, one of the main streets in downtown, ‘Rue du Marais’, literally means, “Street of the Swamp” and the name of the city itself comes from the old Flemish word Broekzele, which roughly translates to ‘settlement in the swamp.’ So, when one talks politically of draining the swamp, at least in Brussels, it carries a literal interpretation that is often distant from the current political climate in DC.
What goes without question is that both Brussels and DC are currently heaving under waves of increasing populist threats to drain political swamps. With the Brexit referendum followed by the election of President Trump, we also now have the Yellow Vest protests in France coupled with a letter accusing President Macron of treason signed by a dozen French generals and a former Minister of Defense. There is an air of anger against political institutions on both sides of the Atlantic, and increasingly, the heated rhetoric is being pointed at the price of medicines.
Investing in EU Biotech IP – What Works?
Vital Transformation released its newest research at the European Health Forum Gastein on October 4th, 2018. Our project analysed 116 EU biotech start-ups from the period of 2001 – 2007 through September 21, 2018 from the UK, Netherlands, Belgium, and Spain. We focused on companies developing medicinal products for human consumption. In this work, we compared the impact of IPOs (stock market listings), private investors, and direct EU funding on their success or failure.
VITAL HEALTH PODCASTS
Our podcast series is also available via your favourite channels!
 |
 |
|---|---|
 |
 |
NEWSLETTER
Register now to receive all the latest updates from Vital Transformation including our research, podcasts and more…
VT on X
Claims that the IRA wouldn’t affect innovation overlooked how early-stage investors really make decisions. Our research shows the pipeline for senior-focused therapies is already narrowing.
— VitalTransformation (@VitalTransform) April 18, 2025
Read the full breakdown: https://t.co/4EzJGdMcU4@weworkforhealth #IRA #DrugPricing
The IRA’s 9-year cap for small molecules and 13-year cap for biologics rerouted investment away from the pills seniors rely on. We sat down with @steveusdin1 to unpack how this shift skews R&D incentives and future therapies.
— VitalTransformation (@VitalTransform) April 27, 2025
Listen here: https://t.co/FCjKFdIhsU@weworkforhealth pic.twitter.com/phoDI4ZrhK
Our clients include many of the world’s leading health care organisations.